Biquelle® XL meets all the relevant regulatory requirements of a bioequivalent medicine (UK reference product Seroquel® XR (quetiapine) 50mg, 150mg, 200mg, 300mg and 400mg prolonged-release tablets [Luye Pharma, London, UK]).1 Four bioequivalence clinical studies were performed.1 One single-dose, randomized crossover study compared Biquelle XL 50mg prolonged-release tablets with Seroquel XR 50mg prolonged-release tablets under fasted conditions. Two single-dose, randomized crossover studies compared Biquelle XL 200mg prolonged-release tablets with Seroquel XR 200mg prolonged-release tablets under fed and fasted conditions.1 One steady-state study compared Biquelle XL 400mg prolonged-release tablets with Seroquel XR 400mg prolonged-release tablets under fasted conditions.1
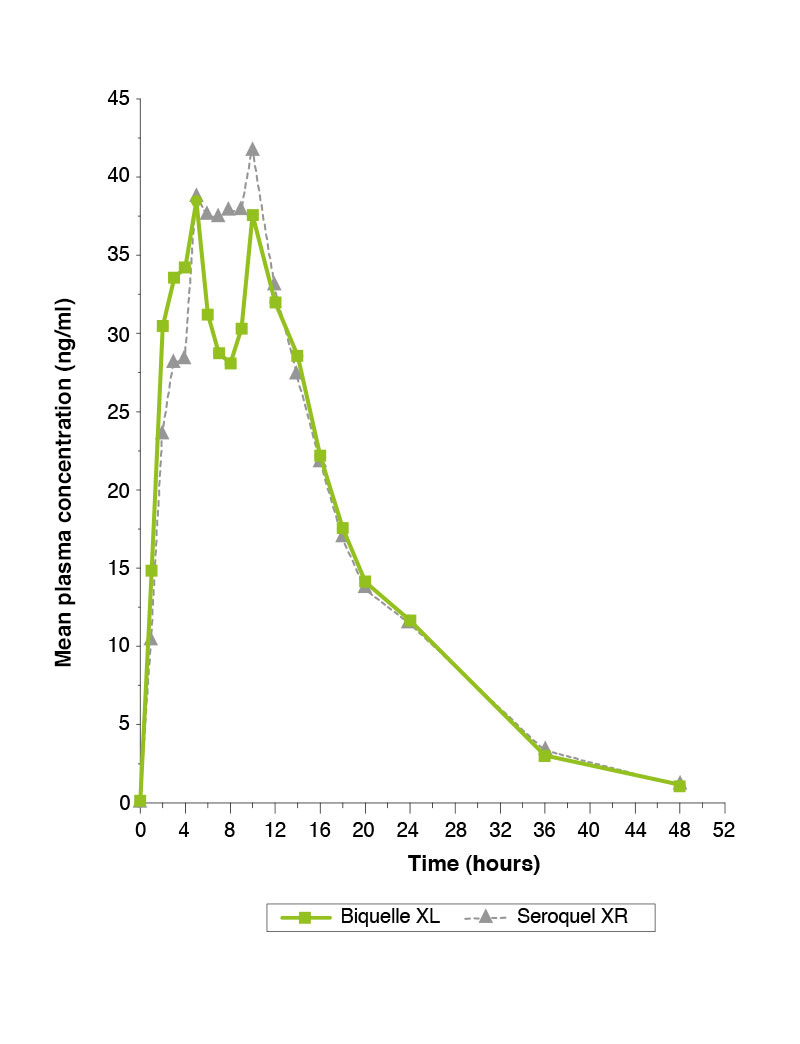
Single-dose study comparing Biquelle XL 50mg and Seroquel XR 50mg under fasted conditions.1
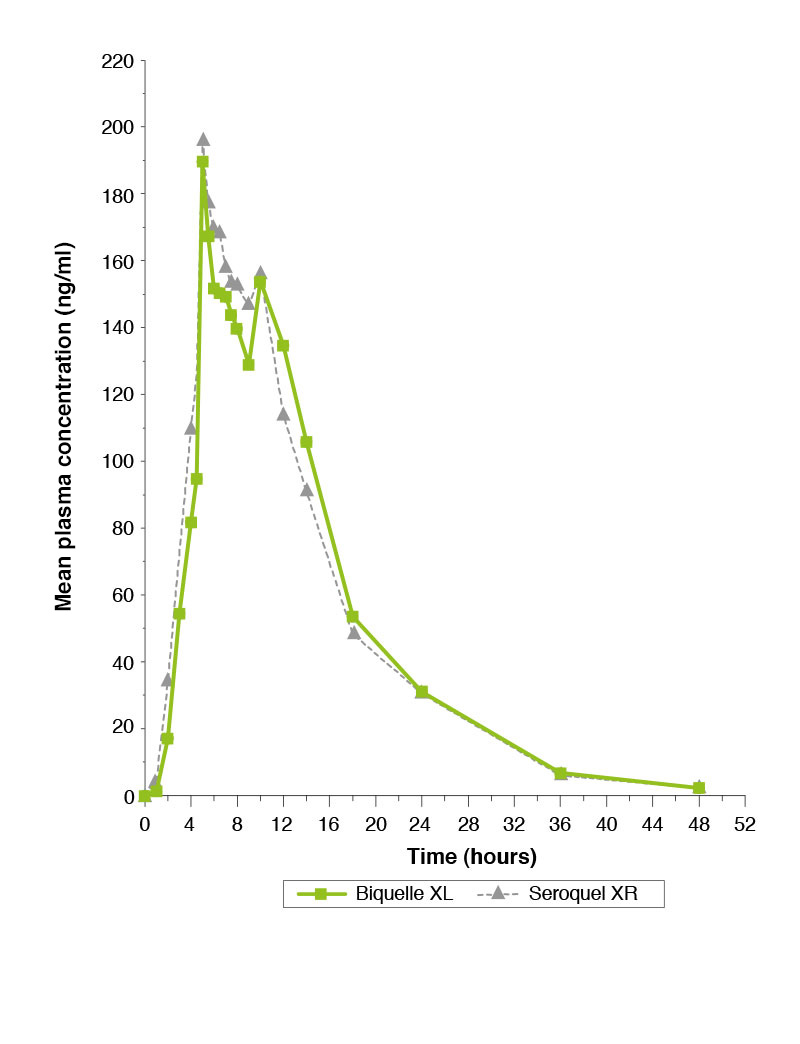
Single-dose study comparing Biquelle XL 200mg and Seroquel XR 200mg under fed conditions
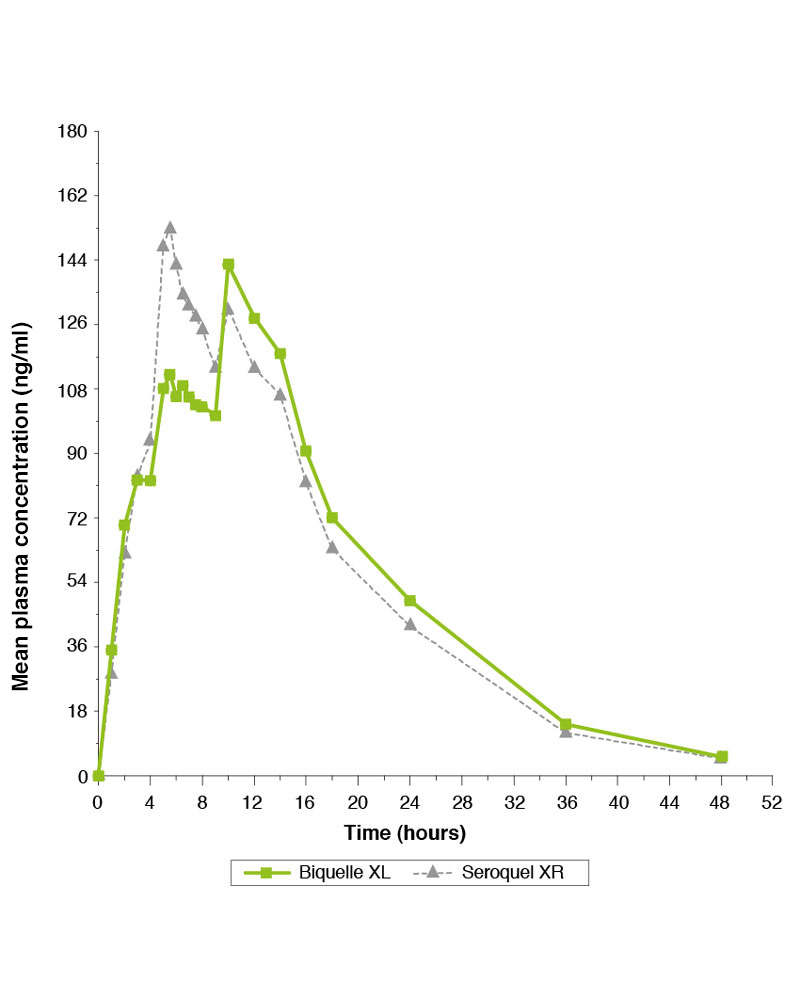
Single-dose study comparing Biquelle XL 200mg and Seroquel XR 200mg under fasted conditions
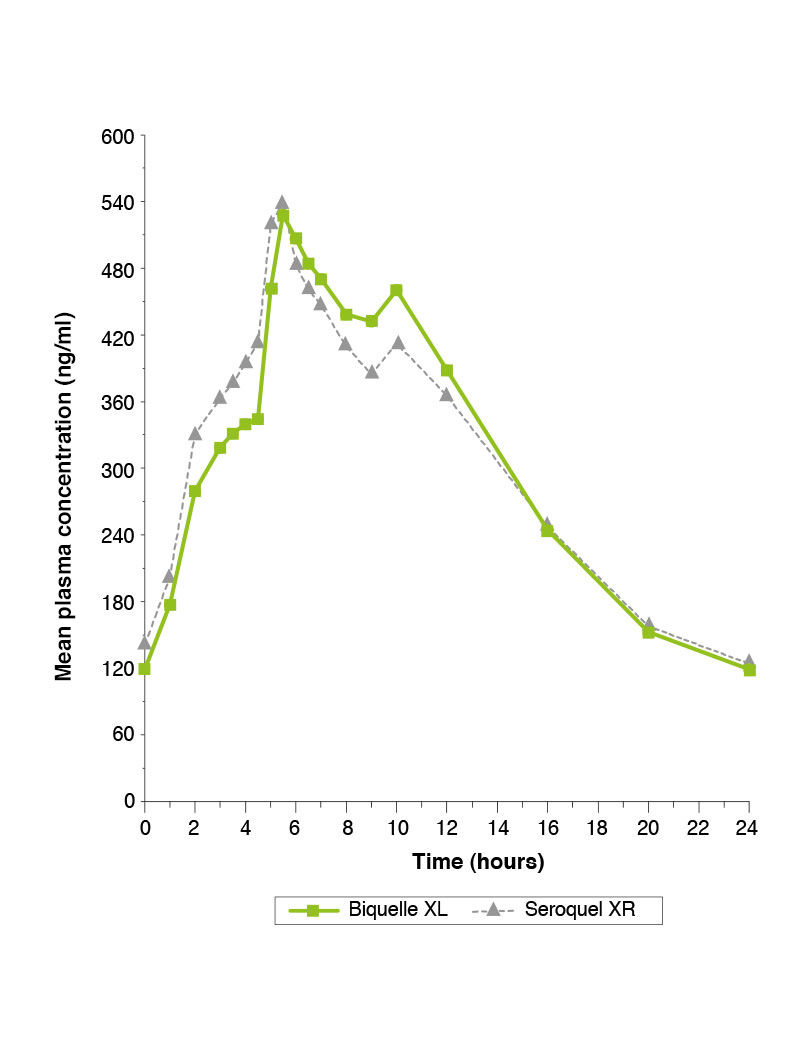
Steady-state study comparing Biquelle XL 400mg and Seroquel XR 400mg under fasted conditions
References: 1) Data on file. 1010067149 v 5.0 September 2021.
BIQ1010269D1_APR2023
















